On
the Origins of the Angiosperm and the Gymnosperm
By Harry
Levin
Copyrighted
Essay Number Three
Abstract
The angiosperm
phylum of the plant kingdom – the
flowering plant – originated in Gondwanaland during the late Devonian,
more than 360 million years ago.
This overarching hypothesis is founded
on late-Paleozoic and Mesozoic plate tectonics and climatology here in
correlation with the past and present geographic distributions of
living plants. It is firmly supported by plant molecular genetics,
plant morphology, and paleoentomology.
Following the saga of five plant families – three angiosperms and two
gymnosperms – the essay illuminates late-Paleozoic to present-day
consociations of angiosperms and gymnosperms in close correlation with
late Paleozoic and Mesozoic plate tectonics and climatology. It
emphasizes the essential late Paleozoic role of insects in evolution of
the bisexual flower. Together with molecular genetics, it finds that
angiosperms and gymnosperms are distinct and separate phyla in coeval
existence since the Devonian; and it contends that both phyla are
monophyletic. Significantly, this essay approximates – with examples –
hitherto unknown dates of origin of individual plant and animal
families.
Throughout this account, the time 280 Ma was momentous – a rapid
pulsing of biology and geology:
• It marked the beginning of the Permian.
• It marked the end of the Permo-Carboniferous
Ice Age, except for a
continuation of
orogenic ice cover extending from
southern Antarctica into the New
South Wales
region of Australia until 250 Ma.
• It marked the water-shed biological
disconnect of Australia from
Gondwanaland.
• It marked the drifting away of a
superterrane, here called Austosunda,
from westernmost
Australia and northern
Gondwanaland – part to eventually settle down
as continental
core of the plateau region above
India and part to become most of
Myanmar (Burma).
1
Prologue: Molecular
Genetic Support
of Devonian Angiosperm Origin
First and foremost, the hypothesis of Devonian
angiosperm origin is
consistent with findings of molecular studies of plant DNA in genetic
analysis. Certain of these studies below imply an early divergence of
angiosperms from a Devonian plant group (probably a seed fern).
• A. V. Troitsky et al. (1) indicate (in 1991) by
rRNA sequence
comparisons that both gymnosperms and angiosperms are monophyletic
groups. The genealogical splitting of gymnosperm and angiosperm
lineages occurred at least 360 million years ago. Magnoliales were the
earliest angiosperms. They are dicotyledons.
• W. F. Martin et al. (2) indicate (in 1993), by
chloroplast and nuclear
sequence data taken together,
that angiosperms and gymnosperms were
separate lineages about 330 million years ago and that the
separation of monocotyledonous and dicotyledonous lineages of
angiosperms took
place about
300 million years ago.
• T. Kh. Samigulin et al. (3) indicated (in 1999),
with partial sequences
of the ropC1 gene, that both angiosperms and gymnosperms are
monophyletic and that none of the recent gymnosperms is sister to the
angiosperm.
• S-M Chaw et al. (4) indicate (in 2000), with
mitochondrial subunit rRNA
sequences, that gymnosperms are monophyletic; that, in conjecture, the
angiosperms too are monophyletic and substantially older than the
fossil record indicates.
• L.M. Bowe et al. (5) indicated (in 2000), with
sequence data of
evolving mitochondrial genes, cox1 and atpA, that extant gymnosperm
genes are monophyletic and that angiosperm origin should be sought
among extinct seed plant groups.
The above molecular genetic papers suggest angiosperm monophylesis.
They are concordant with the forthcoming hypothesis of this essay that
the angiosperm originated in the late Devonian more than 360 million
years ago, – a thesis founded here on late Paleozoic and Mesozoic
plate tectonics and climatology in correlation with the past and
present geographic distributions of living plants.
The above-cited phylogenetic papers refute the Cretaceous theory of
angiosperm origin. The Cretaceous theory professes the abrupt
appearance of a diverse angiosperm flora in the Cretaceous fossil
record of the northern hemisphere, with fallacious implication of
polyphylesis. Moreover, here are cited specific genetic
refutations of the anthophyte theory (6), which would link angiosperms
with the gymnosperm order Gnetales (7) (8) and (indirectly) other
theories which tentatively link the angiosperm bisexual flowers to
conifers. Such theories could imply angiosperm origin in the early
Mesozoic.
However, it must be said that there is not as yet (in 2007) full
acceptance of the molecular genetic theory of the angiosperm as
late-Paleozoic “sister to the gymnosperm.”(9)). Indeed, R.M. Bateman et
al. (10) sum up (in 2006) that while most molecular genetic studies
indicate that all extant gymnosperms form a natural group, “suggesting
early divergence of the lineage that led to angiosperms”; yet “it is
possible that extinct gymnosperms gave origin to the angiosperms”. The
only extinct gymnosperms may have been the Glossopteridales
(Permian).
2
Part I: Evidence of Early Origin of Angiosperms
Assemblage of Five Plant Families
Evidence is presented that early angiosperm and gymnosperm families
were extant more than 300 million years ago over a vast southern
hemisphere supercontinent, Gondwanaland, comprising South America,
Africa, India, Antarctica, Australia, and smaller terranes.
For this endeavor, five plant families are selected, three of
angiosperms, two of gymnosperms, which today have remarkably similar
highly disjunctive southern hemisphere distributions. These are trees
and shrubs that consociate with each other, in southernmost South
America as well as in the South Pacific regions of Australia, New
Zealand and New Caledonia – on islands and on continents nine thousand
kilometers apart, on both flanks of Africa. A key observation,
detailed below, is the near-complete absence of these five exemplar
plant families from Africa.
These are:
• the angiosperm family Proteaceae
• the angiosperm family Winteraceae
• the angiosperm family Nothofagaceae
• the gymnosperm family Araucariaceae
• the gymnosperm family Podocarpaceae
The angiosperms Proteaceae, Winteraceae, and Nothofagaceae have basal
dicotyledons, indicative of a dicot nature of very early angiosperms.
Interestingly, the foliage of Proteaceae, Araucariaceae, and
Podocarpaceae reveals a transition from broad-leafed to needle species.
These trees and shrubs display a sequence of evolutionary curtailment
of leaf form and structure indicative of defensive adaptation to
gradual but varied encroachments of ice fields as probable cause.
Phylogenetic information and present-day geographical distribution of
these southern hemisphere exemplar families are provided in Table I (at
end of essay).
Supercontinental Continuity
In hypothesis, all five floras more than 300 million
years occupied the whole of the southern hemisphere supercontinent
Gondwanaland which was in continuity from South America to Australia.
The first to discern this continuity was J. D. Hooker (11) about 1860.
A notable contemporary of Darwin, Hooker believed that the evidence
of similar flora on widely separate lands could not be explained
without supposing that these lands once formed a continuous expanse (on
the basis of his 1839-1843 voyages and observations with the H. M.
Discovery ships Erebus and Terror). Gondwanaland had not been conceived
of by 1860. By 1970, V.K. Rao said of Proteaceae, on observing their
similarity in far-apart locations (12):
“The similarity in plant associations and the component floristic
elements in the two [Australia and Africa] widely separated
geographical regions presupposes the existence of similar edaphic and
climatic conditions not only at the present time but also in the
historic past and through the period of their evolution. …. The
conclusion that Proteaceae originated on a connected southern continent
which subsequently fragmented, therefore, seems to be
unassailable.”
Throughout earth history, it was almost axiomatic that
continents did
not move. It was not until 1971 that the concept of
continental drift emerged from heresy to first principles. It was
then that the concept of a unified Gondwanaland – consisting of
South America, Africa, Arabia, India, Australia, and Antarctica,
as well as smaller tectonic units – became generally acceptable.
Today sequences of rock that were laid down over a
500-million-year period – before Africa and South America separated
– are almost identical on both sides of the rift, in continents
now some 5,000 km apart. At the start of the Permian 280 million years
ago, Madagascar was attached to India and was near to Africa.
Moreover, India, Africa, and Antarctica were either attached or in
close proximity to each other through Australia.
To emphasize, all of the above five flora more than 300 million years
ago are inferred here to have occupied the whole of the southern
hemisphere supercontinent Gondwanaland, which was continuous from South
America through Australia.
3
The Permo-Carboniferous Ice Age
The presence of the three angiosperms and the two
gymnosperms 300
million years ago throughout Gondwanaland is strongly indicated by the
great effect of a momentous glaciation, the Permo-Carboniferous Ice
Age, also termed the Gondwanaland Ice Age. In hypothesis, this
glaciation is a prime cause of the similarly disrupted distributions of
these five families.
Beginning 300 million years ago, and lasting 50 million years, the
Permo-Carboniferous Ice Age dwarfed recent glaciations, like those of
the Pleistocene. The incursions of the Permo-Carboniferous ice fields
destroyed all life in their paths without trace. The movements of the
ice fields are described by D.H. Tarling and M.P. Tarling (13). In
initial onslaughts, Southern Africa and Southeastern South America were
engulfed. A huge white salient reached into Central Africa; and in
South America, ice cover extended into the lower part of Brazil. Only
the flora to the north, beyond the ice fields, survived. Not all of
Gondwanaland was glaciated. The absence of glacial rubble today in
Northeastern Australia and in some areas of Southwestern Australia
indicates that these areas had eluded the polar ice sheet. This absence
could account for the survival of Australian Proteaceae and other
Gondwanaland angiosperms and gymnosperms despite the pervasive cold.
Today, more than half of all genera of Proteaceae are in Eastern
Australia, and about a third of are in Southwestern Australia.
In hypothesis, the three angiosperm and two gymnosperm families cited
above were present during the Carboniferous prior to the ice invasion,
and before 300 million years ago, over a continuous Gondwanaland
expanse stretching from Australia to South America. Africa had been a
main link along that route of propagation.
In Africa, the Permo-Carboniferous Ice Age devastated each of the
five angiosperm and gymnosperm families cited above. This
imprint of the glaciation is enumerated below as it persists today:
For Proteaceae in Africa, the phylogenetic composition was altered from
14 tribes to essentially one tribe, the Proteeae.
• For the Nothofagaceae in Africa, all traces were
removed.
• For the Winteraceae in Africa, all traces were
removed.
• For the Araucariaceae in Africa, all traces were
removed.
• The Podocarpaceae in Africa were wiped out; but
they made a
limited comeback along the
southeast coast of Africa from Kenya to
Zimbabwe, probably radiating by
land bridge from Madagascar, as
the climate warmed following the
ice age.
The presence of these
five families on both flanks of Africa combined
with African absences or stark alterations asseverate that these
angiosperms and gymnosperms had in the far distant past spread out over
the entire Gondwanaland supercontinent; had existed there 300 million
years ago; and were largely removed from Africa by the ice age of 300
to 250 million years ago.
4
Part II: Additional Evidence of
Angiosperm Antiquity
Fragmentation and Redistribution of Gondwanaland
Gondwanaland began breaking apart during the late
Paleozoic and the Mesozoic. In particular, fragments
(tectonostratigraphic terranes or “megashards”) rifted away from
northwest Australia and drifted north, ultimately to become attached to
southeastern Laurasia. For instance, Ian Metcalfe (14) in 1988
described a group of elongated subcontinental-sized fragments, herein
termed “Austosunda,” which had rifted from Northwest Australia. This
breakaway occurred during the Permo-Carboniferous. Austosunda would
later become part of the continental core of southeastern Asia and
Indonesia. The component blocks of Austosunda are termed Lhasa,
Changtang, and Sibumasu. The Lhasa and Changtang components of
Austosunda became a long southern edge of Laurasia before the coming of
the Indian Plate. Today Lhasa and Changtang together comprise the
Tibetan Plateau. The Sibumasu component of Austosunda is now the
western edge of the Indochina subcontinent. See Figure 1
|
FIGURE 1
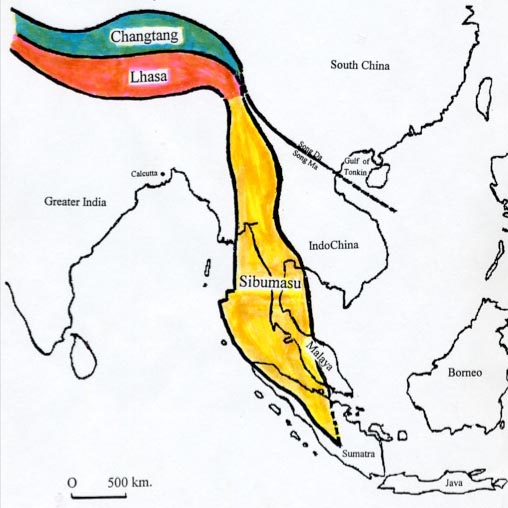 |
Charting of three
continental cores of Southeast Asia: Sibumasu, lhasa, and
Changtang, formerly components of the Austosunda that biolink rifted
from Australia and the start of the Permian 280 million years ago to
become southeastern margins or Laurasia. The Indian Plate Collided with
Laurasia broadly and the Lhasa site to create the Himalayas.
The chart provides
selected elements of Figure 1 of I Metcalfe, "Origin and Assembly of
South-east Asian Continental Terranes," p. 102 in Gondwanaland
and Tethys, ed. by M. G.
Audley-Charles and A. Hallam, Oxford University Press (1988).
|
5
I. Metcalfe (14)
wrote that “by latest early Permian
times,” the
Sibumasu component of the megashard had broken away from Australia.
He
noted: “It is considered here that Sibumasu, [and the Lhasa and
Changtang regions of Tibet] . . . were in continuity and rifted
together
at the same time from Gondwanaland.”
Today flora and fauna of Australia and Sibumasu show similarity along
ancient family lines. Metcalfe cited the early-Permian identity of
biological species on both sides of the break as evidence of a
previously unified biology. He pointed to “late Carboniferous-early
Permian glacial-marine diamictites, cool-water faunas, absence of warm
climate floras, and presence of faunas of Gondwanaland (north-west
Australian) affinities” – in both Australia and continental core
regions (formerly Austosunda) in southeastern Asia. And in his
summary abstract he wrote: “Late Carboniferous and Lower Permian
sediments of the Sibumasu, Lhasa, and Changtang blocks . . .
include
[these] extensive glacial-marine deposits.”
In hypothesis, separation of a connected group of megashards
(Austosunda) from northeastern Gondwanaland occurred at the beginning
of the Permian about 280 million years ago; and Austosunda arrived at
its destination in southeastern Asia near the end of the Jurassic
(about 145 million years ago).
These dated events
(supported throughout these essays) resulted in the
introduction of angiosperms into Laurasia (Eurasia) for the first time.
Hence, this chronology implies that angiosperms are
at least 280
million years old (consistent with the Gondwanaland angiosperm and
gymnosperm antiquity presented above); and, furthermore, it explains
their rather sudden appearance in the northern hemisphere Cretaceous
fossil record.
By middle-to-late Cenomanian, 95 to 91 million years ago, the
angiosperms, dicots and monocots, were the prevalent forms of
vegetation of the northern hemisphere. In further hypothesis, the early
known angiosperms in the northern hemisphere are consistent with
the arrival of the Austosunda terranes 145 million years ago.
The earliest evidence of the presence of angiosperms in Laurasia was
reported by Ge Sun et al. in ~1998 (15). They detail the discovery of
fossil angiosperm fruiting axes in the Yixian Formation in Liaoning
Province in northeast China. The authors dated their find to be about
140 million years old, consistent with the chronology stated above.
In hypothesis, attachment of the Indian Plate to the Eurasian Plate
occurred later, about 85 million years ago during the late Cretaceous,
– 60 million years after the arrival of Austosunda on the shores of
southeast Laurasia. It brought to Eurasia a second biotic cargo. The
Indian Plate became the second center of diversification and radiation
of angiosperms into the northern hemisphere. Angiosperms soon became
the dominant plants upon the land.
6
Solution to Wallace
Line
and Other Disjunction Enigmas
The Sunda Islands are politically a part of Indonesia that extends
toward New Guinea from the Malay Peninsula to the Moluccas. They
include Sumatra, Java, Borneo, Sulawesi, Bali, and Lombok, and numerous
small islands. The Sundas have presented a puzzle of partial-to-total
separation of fauna and flora of Sibumasu and Indian Plate derivation.
On Borneo the native biota is termed “Cathaysian,” brought by the
Indian Plate. To the west, across the straits, the native biota of
Sumatra is termed “Australasian,” brought by Austosunda from
Gondwanaland.
As noted above, many angiosperm families reached Laurasia for the first
time on the Indian Plate, at its later junction with Laurasia, 85
million years ago. By their examples, some families show
phytogeographic disjunction (bimodal distribution patterns) that has
resulted from the two separate debarkations – 60 million years apart –
on the shores of Laurasia. The
present Australasian and Cathaysian
flora and fauna are the respective manifestations of this disjunctive
geography. Their adjacent presences have often been delineated
with no
little wonder ever since noted by Alfred Russell Wallace and are termed
a “Wallace Line” phenomenon.
These biogeophysical accounts of the migrations of Austosunda and the
Indian Plate provide solution to other persistent and troublesome
phytogeographic puzzles. Two prominent puzzles in plant geography and
their solutions are illustrated as follows:
Puzzle 1: the
disjunctive distribution patterns of Nothofagaceae (the
southern beech) on the one hand and the Fagaceae (the beech and the
oak) on the other. All are of the order Fagales.
Solution: The
Nothofagaceae (now with but one genus, Nothofagus) are a Gondwanaland
family that are more than 300 million years old. They consociated
with the Proteaceae in a cold temperate environment before and
during the Gondwanaland Ice Age. Like the Proteaceae, Nothofagaceae
were present in Australia and Austosunda when their separations from
Gondwanaland occurred about 280 million years ago. The present-day
geographic distribution of Nothofagaceae is mainly temperate-zone
southern hemispheric. There are 20 species in New Caledonia and 9
species in South America, 9000 km. away.
On the other hand, the Fagaceae (oak and beech) are a younger family,
which evolved after Austosunda
separation. Oak and beech may have
originated on the Indian Plate. They were ferried on the Indian Plate;
and from there 85 million years ago, they radiated throughout
Laurasia. The Fagaceae are almost entirely northern hemispheric. None
is native to Australasia.
Puzzle 2: the
disjunctive distribution patterns of the Magnoliaceae and
the Annonaceae on the one hand and the Winteraceae and Eupomatiaceae on
the other. All are probably of the order Magnoliales.
7
Solution:
The Winteraceae and the Eupomatiaceae are described as among the
most ancient of the angiosperm families, more than 300 million years
old. Like the Nothofagaceae and the Proteaceae, they were native to
Gondwanaland, including Austosunda and Australia, at the time of the
separation of Australia from Gondwanaland, 280 million years ago. Some
Winteraceae remained in Gondwanaland and in Australia, while others
were ferried on the Austosunda to the margins of Laurasia where it made
landfall 145 million years ago. They are today almost entirely
indigenous to the southern hemisphere (with a small exception of the
Winteraceae genus Drimys), and mainly to Australasia. The Winteraceae
have been cited by Whitmore (16) as, “the southern counterpart to
Magnoliaceae . . . The [Magnoliaceae] distribution contrasts
strongly
with that of Winteraceae.”
On the other hand, the Magnoliaceae and the Annonaceae are younger
families which came into existence after
the separation of Australia
and Gondwanaland. The Annonaceae, the custard apple family, are
the largest family of the order Magnoliales, with 1100 species. The
Magnoliaceae are temperate zone plants, and the Annonaceae,
mostly tropical. Both the Magnoliaceae and the Annonaceae may
have originated in India.
The absence of these latter two families from Australia, New Caledonia,
Tasmania, and New Zealand strongly indicates that they had not spread
throughout Gondwanaland before the separation of Australia at the start
of the Permian some 280 million years ago. It also strongly indicates
that they were not “on board” Austosunda at its separation from
Australia. Hence, they were not ferried to Laurasia 145 million years
ago. The Magnoliaceae and the Annonaceae came to Laurasia on board the
Indian Plate about 85 million years ago (along with many other families
of angiosperms).
Thus the evidence is pertinent that biogeophysical events – both
separations and new attachments of landmasses– differentiate age of
origin. For instance, the facts that the beech and the oak of family
Fagaceae and the magnolia of family Magnoliaceae are not native to
Australasia point to their origins that occurred less than
280 million years ago.
Moreover, it is noteworthy that, by similar consideration, the ages of
other plant families can be determined, as for example, the family
Cactaceae. It was suggested by P. Maxwell in 1990 (17), in “The
Rhipsalis Puzzle,” that the species Rhipsalis baccifera is among the
oldest of extant cacti. A basis for his suggestion is the unique, wide
distribution of that species. He notes that R. baccifera is not only
endemic to South America and North America but also to Africa,
Madagascar, and Sri Lanka; and he quotes Leon Croizat, 1961 (18): “The
Rhipsalidinae certainly yield in antiquity to no other cactus.”
But importantly, in
dating the origin of Rhipsalis, no native
species is detected here for Australia or New Zealand. DNA and
other
evidence point to the family Portulaccaceae as ancestral to Cactaceae.
Genera of Portulaccaceae also occur today in South America, North
America, Africa, Madagascar, and Sri Lanka.
And furthermore, they occur in Australia and New Zealand, as well.
Hence, Cactaceae are less than 280 million years old; Portulaccaceae
are older than 280 years.
8
280 Ma: the Biolink
Rift of
Australia and Austosunda from Gondwanaland
Each of the following
families is less than 280 million years old:
• Cactaceae
• Cichlidae (fish, in a companion essay)
• Fagaceae
• Magnoliaceae
• Annonaceae
• Placental mammal families (in a companion
essay).
The criterion is that these families have had no Australian history
other than recent; and hence, they are assumed to have been absent from
Australia and Austosunda at the beginning of the Permian, 280 million
years ago.
Thus, this date, 280 Ma,
denotes the earliest date of origin for the
genera of the above. On the other hand, Portulaccaceae,
Nothofagaceae,
Winteraceae, and monotreme and marsupial families, all indigenous to
Australia today, are premised to have originated more than 280 million
years ago.
Australia and Austosunda are estimated here to have broken the
biological link (biolink) to Gondwanaland at the beginning of the
Permian about 280 million years ago. In evidence are the early warming
and attendant rise in sea level in certain regions of western and
northern Australia in the midst of the Permo-Carboniferous glaciation.
In evidence, a momentous sea level rise occurred in the geological
history of Australia about 280 million years ago, at the beginning of
the Permian.
P. V. Rich et al (1985) (19) wrote: “Sometime in the early Permian, the
ice began to shrink [in Australia]. It probably disappeared earlier
from Western Australia than it did from the eastern part of the
continent . . . The seas formed deep bays on the Western
Australia
coastal margin.”
In evidence, Figure 2, a version of the Tarlings’ 300-250 Ma geographic
chart (13), presents a complete isolation by water of Australia from
India and, in fact, from the rest of Gondwanaland. The inference is
here taken that at a time during the Permo-Carboniferous Ice Age,
300-250 Ma, Australia became biologically isolated except for
reptiles capable of long-distance travel over water.
9
FIGURE 2
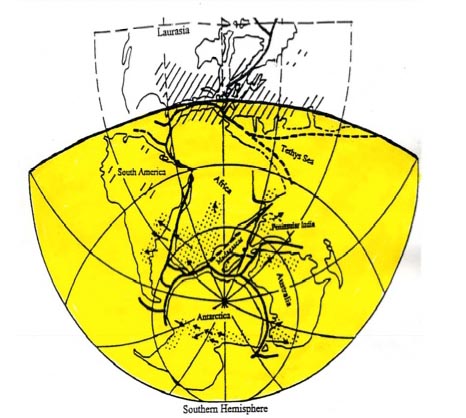 |
Gondwanaland 300 to 250 Million Years Ago
including Ice Field
Incursions during the Gondwanaland Ice Age. Heavy lines make
complete separation by water; broken lines mark incomplete separation.
Source: Tarling and Tarling, Continental
Drift, a Study of the Earth's Moving Surface (1971).
. . . . . . .
. . . . . . .
. . . . . . .
. . . . . . .
|
Areas of glaciation between 250 million
and 300 million years ago with arrows indicating known directions of
ice
movement
|
/////////
////////
|
Areas of tropical coal forests 300
million years ago
|
|
In evidence, M.T. Gibbs et al (2002) (20) wrote: “The
Permian Period . . . contains the most recent transformation from
a major glaciation to
a generally ice-free state . . . Apparently, the deglaciation was
relatively rapid, being mainly confined to the Early Permian Sakmarian
[285-280 Ma] Stage . . . [A Glossopteris
forest cover replaced ice
sheets] and the early and ubiquitous Gandawanan sequence, from tillites
to coal swamp deposits, indicates a major climate warming . . .
”
10
Melville’s “Gonophyll
Theory” of Angiosperm Origin
Ronald Melville (21) reported in 1960: “The primary
diversification of the pre-angiosperm stock appears to have taken
place . . . during the late Carboniferous or early Permian. Many
angiosperm lineages must date back to this period as distinct lines of
evolution . . . ” Melville’s “gonophyll theory” – in explanation of
the evolution of the gynoecium (the female sex organs) and the
androecium (the male sex organs) of the angiosperm flower –
holds that flowers were first built of modified seed fern leaves
bearing either ovules or microsporangia (21).
Although Melville encountered divergence as far back as he was able to
look, he continued: “The great disparity between the floral vascular
systems of Ranunculaceae and Magnoliaceae . . . implies a
very ancient
separation of these stocks. A number of other lineages must be as
old.”
Thus Melville, sought but was unable to establish monophylesis. He was
unable to find the primogenitor, the earliest ancestor of the
angiosperm.
The consequences of Melville’s gonophyll theory conflict with the
generally accepted doctrine of Cretaceous Origin. Hence, Melville’s
gonophyll theory finds itself ignored rather than disproved. However,
the time of origin of angiosperms as conjectured by Melville is in
concord with the time of origin as provided by the hypothesis stated
above.
A chart by P.M. Hurley (22) on the fossil records of Gondwanaland
suggests sufficient time for monophylesis. It shows Gangamopteris (the
seed fern cited by Melville as a possible ancestor of Proteaceae) to
have been present in Eastern Australia during the Devonian 408 to 360
million years ago.
Devonian Co-evolution of
Angiospems and Insects
Insects are here averred to have been essential to the existence of the
angiosperm from the Devonian of more than 360 million years ago until
this day. In the late Paleozoic, the Proteaceae had likely evolved
their nectar-producing glands and nectar reservoirs to attract and
reward pollinators. Such nurturing bounty must well attest that insects
even then were active in fashioning the flowers of the Proteaceae to
their own advantage. An evolutionary reciprocity ensued over many
millions of Paleozoic years in which the Proteaceae induced the
pollinating fauna to co-evolve with them in order for both to utilize
the floral bounty effectively.
The insects were first among faunal pollinators (along with other
arthropods). During the late Paleozoic and thereafter – even in later
times of stress that could mean the sudden absence of a major
cross-pollinating vertebrate – insects might ubiquitously have been
available to carry out pollinations. The earliest traces of insects
have been found in Devonian rocks. Some 800 species of cockroaches
(including winged ones) were extant during the late Carboniferous; and
dragonflies of many sizes were then abundant.
11
Flying beetles, moths, and butterflies came
conspicuously into fossil
record during the Jurassic, which began 208 million years ago; but some
paleontologists note that insects have undergone little change in the
last 200 million years. And the wide diversity of present-day
pollination-specialized insects speaks vividly that long
before the Jurassic, lively adaptations in their form and function took
place. Today, moths and butterflies flit from flower to flower with
body-length proboscises, or sucking tubes, drawn out to drink up
nectar. Today, pollination - specialized scarabs like the African
Trichostetha fascicularis – whose name means “hairy-chest, with hair
in tufts” – move the pollen of Proteaceae effectively from flower to
flower.
Assuredly, in the remote pre-Jurassic past, long before 208 million
years ago, the predecessors of the earliest known moths, butterflies,
and beetles had been induced by entomophilous flowers to evolve bodies
specifically suited to perform cross-pollination. By sustaining the
phylogenetic invention of the bisexual flower, insects have been
essential in fostering the survival of almost-eternal
flowers. They have been the mainstay in pollination of Proteaceae and
other angiosperms from the late Devonian to this day.
In serious error, conventional wisdom abruptly places the origin of the
angiosperm at about 150 million years ago. Such doctrine is utterly
unable to account for the intricate pollination adaptations of
angiosperms and insects – whether it be monocot Orchidaceae with bees
or dicot Proteaceae with beetles. The observation rife among
paleontologists – that insects have undergone little change in the
past 200 million years – stands out in stark contradiction of the
doctrine of 150-million-year-ago origin of angiosperm.
There is no room for doubt that the well-orchestrated, inextricably
complementary forms and functions of insects and flowering angiosperms,
which are manifest in their intricate pollination adaptations, had
co-evolved slowly in the Paleozoic past, long before 150 million years
ago.
Carboniferous Coal Deposits
Consistent with Angiosperm Presence
Intimation of the Carboniferous presence of the
angiosperm and the gymnosperm in Gondwanaland is given in 1996 by R.
Osborne and D. H. Tarling, (23) in describing the formation of late
Carboniferous coal fields. They point out that in North America and
Northern Europe, forests of Lycopsid trees then dominated the swamps
and produced most of the biomass of these coal deposits. (Lycopsids
today are the clubmosses, quillworts, and spikemosses.) On the
other hand, they remark that in Gondwanaland: “A different kind of
coal-forming forest was developing at the same time . . . This
contained entirely different species of vegetation in a cool temperate
climate. The southern flora
contained annual growth rings, showing
large seasonal variations, unlike the tropical forests of the north.”
12
Conclusion
This essay pleads phyletic gradualism.
Charles
Darwin wrote (24) “No complex instinct can possibly be produced through
natural selection, except by the slow and gradual accumulation of
numerous slight yet profitable variations.”
An overarching fundamental thesis is presented here, namely: the
angiosperm originated in Gondwanaland during the late Devonian more
than 360 million years ago.
This thesis is arrived at mainly by
correlation of late-Paleozoic and Mesozoic plate tectonics and
climatology with the present-day geographic distributions of living
plants. It is further attested to by phylogenetic, entomological, and
fossil fuel evidence. The thesis allows sufficient time for origin,
monophyletic diversification, and radiation of angiosperms. It
describes late-Paleozoic consociated radiation patterns of specific
angiosperm and gymnosperm families which exist today.
Component tenets of the thesis are that the fragmentation of
Gondwanaland began in the late Paleozoic and that subsequently (60
million years apart) two huge fragments joined with Laurasia. This
sequence of events offers a criterion hitherto unknown for
approximating and distinguishing the dates of origin of plant and
animal families.
Hence, this essay accounts for the sudden appearance of a diverse
angiosperm flora in the Cretaceous fossil record of the northern
hemisphere. Thereby, it explains the angiosperm diversity
observed in the Cretaceous fossil record, without requiring or implying
polyphylesis.
Table 1: Description of Five S. Hemisphere Exemplar Plant Families
References
1. Troitsky, A.V., Melekhovets, Yu. F.,
Rakhimova, G.M., Bobrova, V. K.,
Valiejo-Roman, K.M., and Antonov, A.S., Angiosperm
origin …. deduced
from rRNA sequence comparisons, J. Mol. Evol.32 (3),
253-61, (1991).
2. Martin, W.F., Lydiate, D., Brinkmann, H., Forkmann, G., Saedler, H.,
and Rudiger, C., Molecular phylogenies in angiosperm
evolution,
Mol.
Biol. Evol. 10 (1), 140-162 (1993).
3. Samigulin, T. Kh., Martin, W.F., Troitsky, A.V., and Antonov, A.S.,
Molecular data from the chloroplast rpoC1 gene
suggest a deep and
distinct dichotomy: gymnosperms (including gnetales)
and
angiosperms,
J. Mol.Evol.49 (3), 310-315 (1999).
4. Chaw, S-M., Parkinson, C.L., Cheng, Y., Vincent, T.M., and
Palmer. J.D., Seed plant phylogeny inferred from all
three plant
genomes, PNAS 97, 4086-4091 (2000).
5. Bowe, L.M., Coat, G., and dePamphilis, C.W., Philogeny of seed
plants
based on three genomic compartments, PNAS 97,
4092-4097 (2000).
6. Doyle, J.A., and Donoghue, M.J., Phylogenies and angiosperm
diversification, Paleobiology 19 (2) 141-167 (1993).
7. Winter, K-U., Becker, A., Munster, T., Kim, J. T., Saedler, S., and
Theissen, G., MADS-box genes reveal that gnetophytes
are more related
to conifers than to flowering plants, PNAS 96,
7342-7347 (1999).
8. Hansen, A., Hansmann, S., Samigullin, T., Antonov, A., and Martin,
W.,
Gnetum and the angiosperms, Mol. Biol. Evol. 16
1006-1009 (1999).
9. Theissen, G. and Melzer, R., Molecular mechanisms underlying origin
and diversification of the angiosperm flower, Annals
of Botany 100 (3)
603-619 (2007).
10. Bateman, R.M., Hilton, J., and Rudall, P.J., Morphological and
molecular phylogenetic context of the
angiosperms, Journal of
Experimental
Botany, 57 (13) 3471-3503, (2006).
11. Hooker, J.D., The botany of the Antarctic voyage of H. M. Discovery
ships Erebus and Terror in the years
1839-1843, under the command of
Captain Sir James Clark Ross, in Flora
Tasmaniae, 3, ~1859.
12. Rao, C.V., Botanical Monograph No. 6, Proteaceae, Council of
Scientific and Industrial Research, New
Delhi, 1971.
13. Tarling, D.H. and Tarling, M.P., Continental
Drift, A Study of the
Earth’s Moving Surface, G. Bell and
Sons, London,
1971.
14. Metcalfe, I., “Origin and assembly of South-east Asian
continental terranes,”
101-118, in Gondwana and Tethys, eds.
M.G. Audley- Charles and A.
Hallam,
Geological Society, Oxford Press, 1988.
15. Sun, G., Ji, Q., Dilcher, D.L., Zheng, S., Nixon, K.C., and Wang,
X.,
Archaefructaceae, a new basal angiosperm
family, Science, 282,
1692-1695,
(1998).
16. Whitmore, T.C., Phytogeography of Eastern Tethys in Wallace’s Line
and Plate Tectonics, ed. T.C. Whitmore,
Claredon Press, Oxford,
1981.
17. Maxwell, P., The Rhipsalis Riddle – or the day the cacti came
down from the trees, New Zealand Cactus
and Succulent Journal,
quarterly,
~1999.
18. Croizat, L., Principia Botanica or Beginnings of Botany,
2 volumes, pp. 758-759, private
publication, 1961.
19. Rich, P.V., van Tets, G.F., and Knight, F., Kadimakara, p.77,
Pioneer Design Studio, Victoria,
Australia, 1985.
20. Gibbs, M.T., Rees, P.M., Kutzbach, J.E., Ziegler, A.M., Behling,
P.J., and
Rowley, D.B., Simulations of Permian
climate and comparisons with
climate-sensitive
sediments, The Journal of Geology, 110,
33-55, (2002).
21. Melville, R., Glossipteridae, Angiospermidae, and the evidence of
angiosperms’ origin, Bull. J. Linn.
Soc., 86, 279-323 (1983).
22. Hurley, P.M., The confirmation of continental drift, 56-67, (1968),
in
Continents Adrift, readings from
Scientific American, W. H. Freeman,
San Francisco,
1971.
23. Osborne, R. and Tarling, D., The Historical Atlas of the Earth,
Henry
Holt and Company, New York, 1996.
24. Darwin, C., The Origin of Species: By Means
of Natural
Selection,
Chapter VIII, Random House and others.
25. Whitmore, T.C., Phytogeography of the eastern end of Tethys,
307-311, in
Gondwana and Tethys, eds. M. G.
Audley-Charles and A. Hallam,
Geological
Society, Oxford Press, 1988.
|
|


